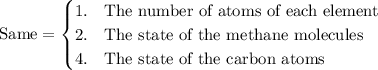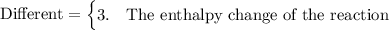Ch4 (g) yields c(g) + 4h (g) (reaction for expansion)
compare the reaction for the "expansion...

Ch4 (g) yields c(g) + 4h (g) (reaction for expansion)
compare the reaction for the "expansion" of methane with the reverse of the reaction that represents the standard enthalpy of formation. which properties are the same for both reactions and which are different?
1. the number of atoms of each element, 2. the state of the methane molecules, 3. the enthalpy change of the reaction, 4. the state of the carbon atoms.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 10.02.2021 23:50





English, 10.02.2021 23:50

Mathematics, 10.02.2021 23:50

Mathematics, 10.02.2021 23:50

English, 10.02.2021 23:50