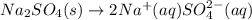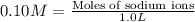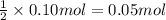Chemistry, 29.06.2019 02:20 SophieStar15
Sodium sulfate dissolves as follows: na2so4(s) → 2na (aq) so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
Sodium sulfate dissolves as follows: na2so4(s) → 2na (aq) so42- (aq). how many moles of na2so4 are...
Questions in other subjects:

Physics, 24.08.2019 06:00


Mathematics, 24.08.2019 06:00

Mathematics, 24.08.2019 06:00


Business, 24.08.2019 06:00


Mathematics, 24.08.2019 06:00


Chemistry, 24.08.2019 06:00