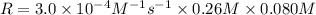Chemistry, 28.06.2019 23:30 springcoates
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no2–]. at 25ºc, the rate constant is 3.0 × 10–4/ m · s. calculate the rate of the reaction at this temperature if [nh4+] = 0.26 m and [no2–] = 0.080 m. (5 points)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no...
Questions in other subjects:


Mathematics, 23.08.2021 18:10

Biology, 23.08.2021 18:10

Mathematics, 23.08.2021 18:10

Biology, 23.08.2021 18:10


Chemistry, 23.08.2021 18:10



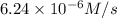 .
.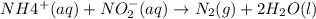
![[NH_4^{+}]=0.26 M](/tpl/images/0028/9019/f2f2d.png)
![[NO_2^{-}]=0.080 M](/tpl/images/0028/9019/214e5.png)
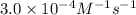
![R= k[NH_{4}^+][NO_{2}^-]](/tpl/images/0028/9019/cb361.png)