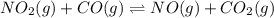Chemistry, 28.06.2019 21:20 tamikalove78
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume of the system is decreased at constant temperature, what change will this cause in the system? a shift to produce more no a shift to produce more co a shift to produce more no2 no shift will occur

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume...
Questions in other subjects: