
Chemistry, 28.06.2019 20:20 joseroblesrivera123
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) 2zn (s) + h+ (aq) → 2zn2+ (aq) + h2 (g) zn (s) + 2hbr (aq) → znbr2 (aq) + 2h+ (aq) zn (s) + 2br- (aq) → znbr2 (aq) zn (s) + 2hbr (aq) → znbr2 (s) + 2h+ (aq)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ion...
Questions in other subjects:

Mathematics, 28.07.2019 18:00


Spanish, 28.07.2019 18:00





Biology, 28.07.2019 18:00


English, 28.07.2019 18:00

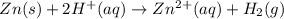
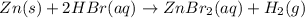

 ion is the spectator ions.
ion is the spectator ions.


