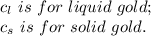Chemistry, 28.06.2019 18:30 ilizzy1224
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg of liquid gold at an initial temperature at 1336k. the solid and liquid other. when the two reach thermal equilibrium will the mixture be entirely solid, or will they be in a mixed solid/liquid phase? explain how you know. draw two separate temp. vs. energy added diagrams to you answer this question. can only exchange heat with each

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2


Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg...
Questions in other subjects:





Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01

History, 02.10.2020 19:01



Mathematics, 02.10.2020 19:01