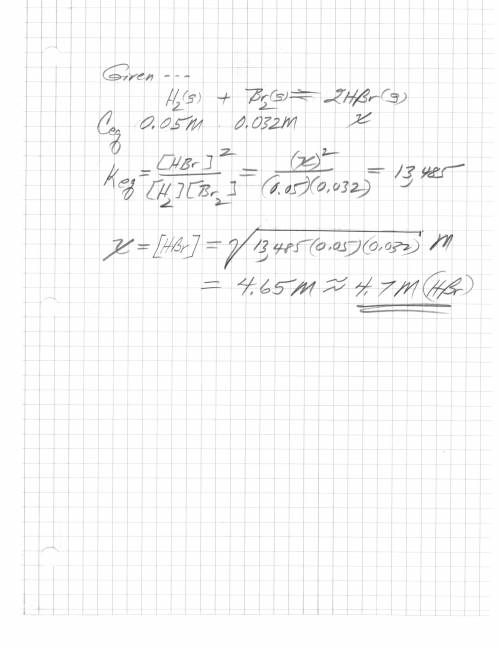
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium...

Chemistry, 23.11.2019 20:31 abronxtale02
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium the h2 concentration is 0.05 m, while the br2 concentration is 0.023 m. calculate the hbr concentration at equilibrium, to 1 decimal. be careful with the units.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
Questions in other subjects:

Biology, 11.05.2021 20:00


Mathematics, 11.05.2021 20:00

Biology, 11.05.2021 20:00

Mathematics, 11.05.2021 20:00

Mathematics, 11.05.2021 20:00


Computers and Technology, 11.05.2021 20:00


Biology, 11.05.2021 20:00