
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper sulfate pentahydrate is 249.68 g/mol, calculate grams of copper sulfate pentahydrate that will dissolve in 100 g of water at 0oc (show calculations for full credit)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper...
Questions in other subjects:


Social Studies, 01.05.2021 05:20

Mathematics, 01.05.2021 05:20


Mathematics, 01.05.2021 05:20



Biology, 01.05.2021 05:20

Mathematics, 01.05.2021 05:20

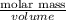
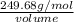
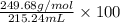
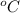 .
. 


