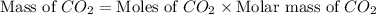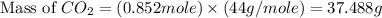Chemistry, 27.06.2019 19:20 robert7248
Given the following equation: 2 c4h10 13 o2 > 8 co2 10 h20 + how many grams of co2 are produced if 12.4 grams of c4h10 reacts with 56.9 grams of o2?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
Given the following equation: 2 c4h10 13 o2 > 8 co2 10 h20 + how many grams of co2 are produced...
Questions in other subjects:

Social Studies, 07.07.2020 22:01

History, 07.07.2020 22:01

Mathematics, 07.07.2020 22:01

Mathematics, 07.07.2020 22:01





Mathematics, 07.07.2020 22:01

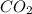 produced will be, 37.488 grams.
produced will be, 37.488 grams.
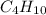 = 12.4 g
= 12.4 g
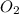 = 56.9 g
= 56.9 g
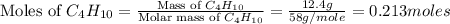


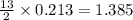 moles of
moles of 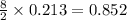 moles of
moles of