

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

You know the right answer?
Consider the following reaction: 2na 2hci > 2n»c1 + h2 how many mols of hydrogen gas (h2) can b...
Questions in other subjects:

Mathematics, 22.10.2019 03:00



Chemistry, 22.10.2019 03:00

Mathematics, 22.10.2019 03:00



Mathematics, 22.10.2019 03:00

Mathematics, 22.10.2019 03:00

Advanced Placement (AP), 22.10.2019 03:00

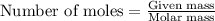 ....(1)
....(1)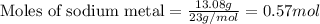
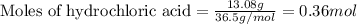
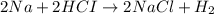
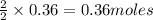 of sodium metal.
of sodium metal.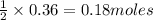 of hydrogen gas.
of hydrogen gas.

