

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
You know the right answer?
Given the following equation: 4 nh3 (g)5 o2 (g) > 4 no (g) + 6 h20 (i) how many moles of nh3 is...
Questions in other subjects:


History, 20.03.2020 22:50





Mathematics, 20.03.2020 22:50



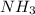 is required to react with 25.7 grams of
is required to react with 25.7 grams of 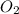 .
.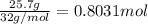
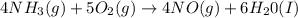
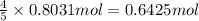 of ammonia gas
of ammonia gas

