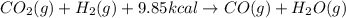Chemistry, 26.06.2019 20:20 Santos7446
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each mole of co2(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each m...
Questions in other subjects:

Social Studies, 17.01.2021 08:30




Business, 17.01.2021 08:30



Business, 17.01.2021 08:30


Mathematics, 17.01.2021 08:30