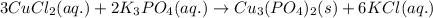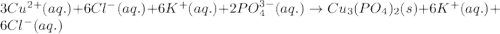Complete and balance the molecular equation for the reaction of aqueous copper(ii) chloride, cucl2, and aqueous potassium phosphate, k3po4. include physical states. molecular equation: cucl2(aq)+k3po4(aq)⟶ enter the balanced net ionic equation for this reaction. include physical states. net ionic equation.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3


Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Complete and balance the molecular equation for the reaction of aqueous copper(ii) chloride, cucl2,...
Questions in other subjects:


Chemistry, 20.04.2021 18:40

Mathematics, 20.04.2021 18:40

Computers and Technology, 20.04.2021 18:40

History, 20.04.2021 18:40