
Chemistry, 26.06.2019 16:10 pchisholm100
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° = ? use the reactions here to determine the δh° for reaction (i):
(ii) hcl(g) ⟶ hcl(aq) δh(ii) ° = −74.8 kj
(iii) h2 (g) + cl2 (g) ⟶ 2hcl(g) δh(iii) ° = −185 kj
(iv) alcl3 (aq) ⟶ alcl3 (s) δh(iv) ° = +323 kj/mol
(v) 2al(s) + 6hcl(aq) ⟶ 2alcl3 (aq) + 3h2 (g) δh(v) ° = −1049 kj

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 21:50, BookandScienceNerd
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
Questions in other subjects:





Biology, 08.03.2021 20:40



Physics, 08.03.2021 20:40


English, 08.03.2021 20:40

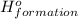 for the reaction is -1406.8 kJ.
for the reaction is -1406.8 kJ.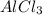 is:
is: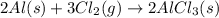

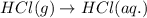
 ( × 6)
( × 6)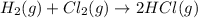
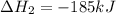 ( × 3)
( × 3)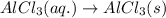
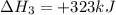 ( × 2)
( × 2)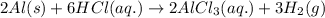
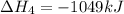
![\Delta H^o_{formation}=[6\times \Delta H_1]+[3\times \Delta H_2]+[2\times \Delta H_3]+[1\times \Delta H_4]](/tpl/images/0020/0754/79ecd.png)
![\Delta H^o_{formation}=[(-74.8\times 6)+(-185\times 3)+(323\times 2)+(-1049\times 1)]=-1406.8kJ](/tpl/images/0020/0754/37adf.png)


