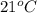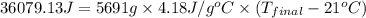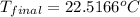Chemistry, 26.06.2019 05:10 kaitlan225
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c...
Questions in other subjects:

Mathematics, 29.06.2019 21:30


Mathematics, 29.06.2019 21:30

History, 29.06.2019 21:30



Mathematics, 29.06.2019 21:30

Biology, 29.06.2019 21:30

English, 29.06.2019 21:30

History, 29.06.2019 21:30

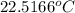
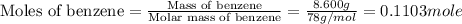
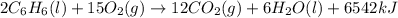
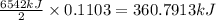 of energy on combustion.
of energy on combustion.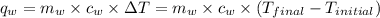
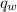 = heat released = 360.7913 kJ = 36079.13 J
= heat released = 360.7913 kJ = 36079.13 J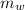 = mass of water = 5691 g
= mass of water = 5691 g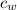 = specific heat of water=
= specific heat of water= 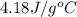
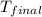 = final temperature = ?
= final temperature = ?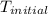 = initial temperature =
= initial temperature =