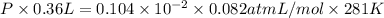Chemistry, 26.06.2019 04:20 silviamgarcia
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid ch3coch3 is placed in a closed, evacuated 360. ml container at a temperature of 281 k. calculate what the ideal gas pressure would be in the container if all of the liquid acetone evaporated.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

You know the right answer?
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid...
Questions in other subjects:


Chemistry, 08.10.2019 22:00


Chemistry, 08.10.2019 22:00



Mathematics, 08.10.2019 22:00



History, 08.10.2019 22:00



 mole
mole