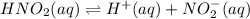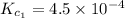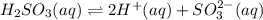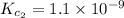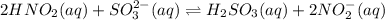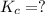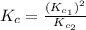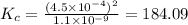Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq)h2so3(aq)⇌⇌h+(aq) + no2−(aq)2h+(aq) + so32−(aq)kc = 4.5 × 10−4kc = 1.1 × 10−9 what is the value of kc for the reaction 2hno2(aq) + so32−(aq)⇌h2so3(aq) + 2no2−(aq)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

You know the right answer?
Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq...
Questions in other subjects:

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

History, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Social Studies, 18.09.2020 01:01

Social Studies, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

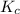 for the final reaction is, 184.09
for the final reaction is, 184.09