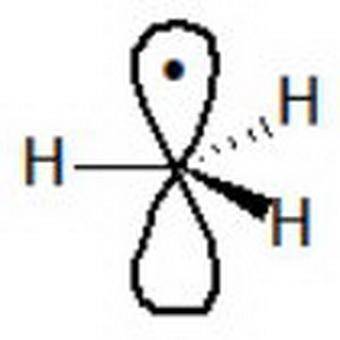
Chemistry, 26.06.2019 03:30 ethanboshears15
Studies indicate that the methyl radical is trigonal planar. based on this, which of the following best describes the methyl radical? the carbon is sp2 hybridized and the unpaired electron occupies an sp2 orbital. the carbon is sp2 hybridized and the unpaired electron occupies a 2p orbital. the carbon is sp3 hybridized and the unpaired electron occupies an sp3 orbital. the carbon is sp3 hybridized and the unpaired electron occupies a 2p orbital.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Studies indicate that the methyl radical is trigonal planar. based on this, which of the following b...
Questions in other subjects:

Mathematics, 27.11.2021 19:20

Mathematics, 27.11.2021 19:20





Social Studies, 27.11.2021 19:20


Mathematics, 27.11.2021 19:20

Mathematics, 27.11.2021 19:20