Chemistry, 23.01.2020 04:31 caitlynnstokes
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using a current of 10.0 a. what mass of copper is deposited in 10.0 minutes? avogadro's number is 6.022 × 1023 molecules/mol and e = 1.60 × 10-19 c.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using...
Questions in other subjects:

History, 03.12.2021 06:50

Computers and Technology, 03.12.2021 06:50

Computers and Technology, 03.12.2021 06:50

Mathematics, 03.12.2021 06:50

History, 03.12.2021 06:50





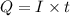
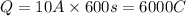
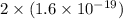
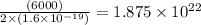 atoms
atoms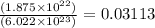 moles
moles