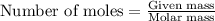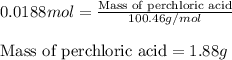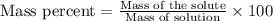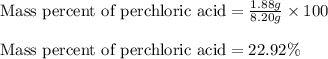Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2



Chemistry, 23.06.2019 05:00, andrwisawesome0
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
A8.20 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. if...
Questions in other subjects:

Mathematics, 05.05.2020 15:46


Chemistry, 05.05.2020 15:46


Mathematics, 05.05.2020 15:46

Mathematics, 05.05.2020 15:46


History, 05.05.2020 15:46

Health, 05.05.2020 15:46

Mathematics, 05.05.2020 15:46

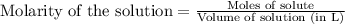
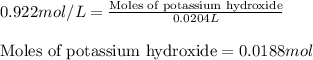
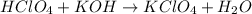
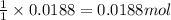 of perchloric acid.
of perchloric acid.