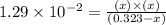Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1


Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
The equilibrium constant, kc, for the following reaction is 1.29×10-2 at 600 k. cocl2(g) co(g) + cl2...
Questions in other subjects:

English, 26.10.2019 22:43

Mathematics, 26.10.2019 22:43




Medicine, 26.10.2019 22:43

Mathematics, 26.10.2019 22:43


Mathematics, 26.10.2019 22:43

Mathematics, 26.10.2019 22:43

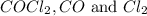 are, 0.2646, 0.0584 and 0.0584.
are, 0.2646, 0.0584 and 0.0584.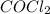 = 0.323 mole
= 0.323 mole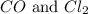 be, 'x'. So,
be, 'x'. So,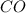 = x M
= x M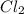 = x M
= x M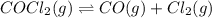
![K_c=\frac{[CO][Cl_2]}{[COCl_2]}](/tpl/images/0436/7314/59c52.png)