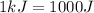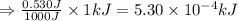Chemistry, 31.01.2020 22:02 ComicSans01
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0.295 gram piece of metal and combine it with 65 ml of 1.00 m hcl in a coffee-cup calorimeter. if the molar mass of the metal is 57.78 g/mol, and you measure that the reaction absorbed 104 j of heat, what is the enthalpy of this reaction in kj per mole of limiting reactant? enter your answer numerically to three significant figures in units of kj/mol.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0....
Questions in other subjects:

Biology, 04.07.2019 17:30

Chemistry, 04.07.2019 17:30

Mathematics, 04.07.2019 17:30

Mathematics, 04.07.2019 17:30


Mathematics, 04.07.2019 17:30



History, 04.07.2019 17:30

Geography, 04.07.2019 17:30

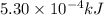
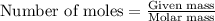 ....(1)
....(1)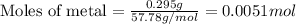
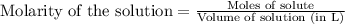
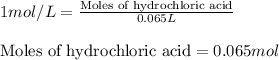
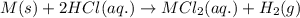
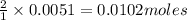 of hydrochloric acid.
of hydrochloric acid.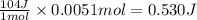 of heat.
of heat.