Chemistry, 28.01.2020 11:31 covergurllaa
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of sulfuric acid (h2so4). what was the molarity of the koh solution if 25.2 ml of 1.50 m h2so4 was needed? the equation is 2koh(aq)+h2so4(aq)→k2so4(aq)+2h2o(l )

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 04:00, clickbaitdxl
What two categories of toxins were present in the air at dish, texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of...
Questions in other subjects:


Mathematics, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40


Mathematics, 11.06.2021 20:40



English, 11.06.2021 20:40

SAT, 11.06.2021 20:40

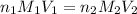
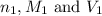 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 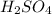
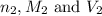 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.