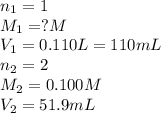Chemistry, 10.01.2020 11:31 mpzpowell7506
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.110 l0.110 l sample of an unknown hno3hno3 solution required 51.9 ml51.9 ml of 0.100 m ba(oh)20.100 m ba(oh)2 for complete neutralization. what is the concentration of the hno3hno3 solution? concentration:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2



Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(a...
Questions in other subjects:

Biology, 23.04.2020 16:18

Mathematics, 23.04.2020 16:18








Mathematics, 23.04.2020 16:18

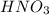 solution will be 0.094 M.
solution will be 0.094 M.
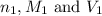 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 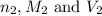 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is