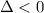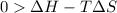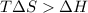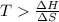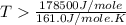For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh∘rxn 178.5kj/mol δs∘rxn 161.0j/(mol⋅k) calculate the temperature in kelvins above which this reaction is spontaneous.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, pinkypie123457
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2



Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variat...
Questions in other subjects:

Mathematics, 02.07.2019 20:30

Mathematics, 02.07.2019 20:30

History, 02.07.2019 20:30

Biology, 02.07.2019 20:30

English, 02.07.2019 20:30


English, 02.07.2019 20:30


Mathematics, 02.07.2019 20:30

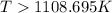
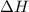 = 178.5 KJ/mole = 178500 J/mole
= 178.5 KJ/mole = 178500 J/mole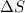 = 161.0 J/mole.K
= 161.0 J/mole.K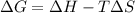
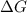 is negative or we can say that the value of
is negative or we can say that the value of