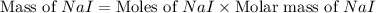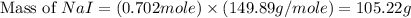Chemistry, 15.11.2019 14:31 ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions in other subjects:

Mathematics, 18.07.2019 15:30



Social Studies, 18.07.2019 15:30

Chemistry, 18.07.2019 15:30

Mathematics, 18.07.2019 15:30




Spanish, 18.07.2019 15:30

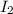 = 89.1 g
= 89.1 g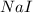 = 149.89 g/mole
= 149.89 g/mole

 moles of
moles of