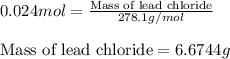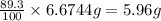An aqueous solution containing 7.96 g7.96 g of lead(ii) nitrate is added to an aqueous solution containing 6.82 g6.82 g of potassium chloride. enter the balanced chemical equation for this reaction. be sure to include all physical states. balanced chemical equation: what is the limiting reactant? potassium chloride lead(ii) nitrate the percent yield for the reaction is 89.3%89.3% . how many grams of precipitate is recovered? precipitate recovered: gg how many grams of the excess reactant remain? excess reactant remaining: g

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:00, jennnifercrd59jc
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
You know the right answer?
An aqueous solution containing 7.96 g7.96 g of lead(ii) nitrate is added to an aqueous solution cont...
Questions in other subjects:

Geography, 27.12.2019 05:31

Health, 27.12.2019 05:31

Geography, 27.12.2019 05:31


Mathematics, 27.12.2019 05:31


Mathematics, 27.12.2019 05:31

Geography, 27.12.2019 05:31

Biology, 27.12.2019 05:31

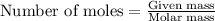 ....(1)
....(1)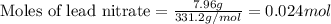
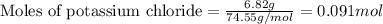
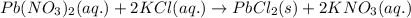
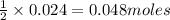 of potassium chloride
of potassium chloride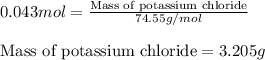
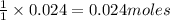 of lead chloride
of lead chloride