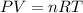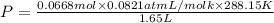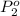Chemistry, 13.10.2019 01:10 sabrinamarie391
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the mole fraction of n2. express your answer using two significant figures. x1 x 1 = nothing request answer part b calculate the mole fraction of o2. express your answer using two significant figures. x2 x 2 = nothing request answer part c calculate the partial pressure of n2. express your answer using two significant figures. p1 p 1 = nothing atm request answer part d calculate the partial pressure of o2. express your answer using two significant figures. p2 p 2 = nothing atm request answer provide feedback

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the m...
Questions in other subjects:

Mathematics, 26.06.2020 16:01



Mathematics, 26.06.2020 16:01






English, 26.06.2020 16:01

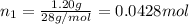
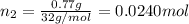

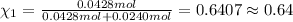

 = 0.0428 mol + 0.0240 mol = 0.0668 mol
= 0.0428 mol + 0.0240 mol = 0.0668 mol