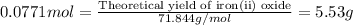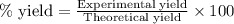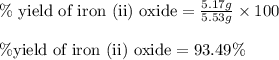Chemistry, 26.10.2019 11:43 Kathryn014
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yields 5.17 grams of iron(ii) oxide . iron ( s ) + oxygen ( g ) iron(ii) oxide ( s ) what is the theoretical yield of iron(ii) oxide ? 21.6 grams what is the percent yield for this reaction ? 85 %

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2


Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yield...
Questions in other subjects:






Mathematics, 09.11.2019 01:31



Mathematics, 09.11.2019 01:31

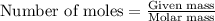 ....(1)
....(1)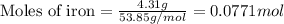
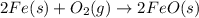
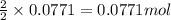 of iron (ii) oxide
of iron (ii) oxide