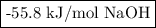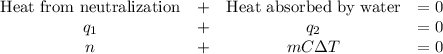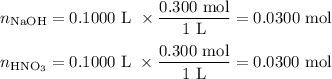A100.0 ml sample of 0.300 m naoh is mixed with a 100.0 ml sample of 0.300 m hno3 in a coffee cup calorimeter. if both solutions were initially at 35.00°c and the temperature of the resulting solution was recorded as 37.00°c, determine the δh°rxn (in units of kj/mol naoh) for the neutralization reaction between aqueous naoh and hcl. assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water. - 34.4 kj/mol naoh -169 kj/mol naoh -55.7 kj/mol naoh -27.9 kj/mol naoh -16.7 kj/mol naoh

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A100.0 ml sample of 0.300 m naoh is mixed with a 100.0 ml sample of 0.300 m hno3 in a coffee cup cal...
Questions in other subjects:




Mathematics, 26.01.2022 14:40




SAT, 26.01.2022 14:50


Mathematics, 26.01.2022 14:50