Chemistry, 24.06.2019 08:00 nancyrj3860
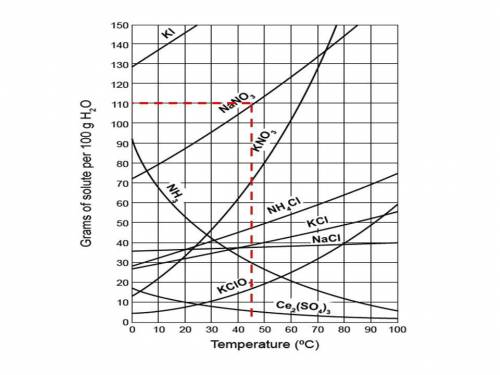
How many grams of nano3 would have to be added to 100. grams of water at 45°c to make a saturated solution of this salt? 1. 100. 2. 110. 3. 120. 4. 130.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
How many grams of nano3 would have to be added to 100. grams of water at 45°c to make a saturated so...
Questions in other subjects:




English, 21.01.2021 23:00