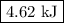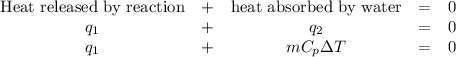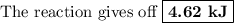When 40.0 ml of 1.00 m h2so4 is added to 80.0 ml of 1.00 m naoh at 20.00°c in a coffee cup calorimeter, the temperature of the aqueous solution increases to 29.20°c. if the mass of the solution is 120.0 g and the specific heat of the calorimeter and solution is 4.184 j/g • °c, how much heat is given off in the reaction? (ignore the mass of the calorimeter in the calculation.) use q=mcp(tiangle)t 4.62 kj 10.0 kj 14.7 kj 38.5 kj

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, bbombard21
Select the atomic models that belong to the same element
Answers: 2

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

You know the right answer?
When 40.0 ml of 1.00 m h2so4 is added to 80.0 ml of 1.00 m naoh at 20.00°c in a coffee cup calorimet...
Questions in other subjects:


Mathematics, 26.09.2019 03:30


Mathematics, 26.09.2019 03:30

History, 26.09.2019 03:30



History, 26.09.2019 03:30


History, 26.09.2019 03:30