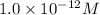Chemistry, 24.06.2019 15:40 dashawn3rd55
The ph of a solution is 2.0. which statement is correct? useful formulas include [h304)= 107, oh-1- 10+00h ph+poh = 14 and (h20-oh-] = 10-14 the poh of the solution is 12.0. the concentration of oh-ions is 1.0 x 10-2 m. the concentration of h3o+ ions is 100.0 m. the poh of the solution is 16.0.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
The ph of a solution is 2.0. which statement is correct? useful formulas include [h304)= 107, oh-1-...
Questions in other subjects:

Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00

Biology, 23.02.2021 01:00

Mathematics, 23.02.2021 01:00

Biology, 23.02.2021 01:00

Chemistry, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00

Business, 23.02.2021 01:00

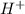 concentration.
concentration.
![pH=-\log [H^+]](/tpl/images/0012/3146/37e81.png)
![2=-\log [H^+]](/tpl/images/0012/3146/97dc6.png)
![[H^+]=0.01M](/tpl/images/0012/3146/8ae83.png)
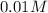
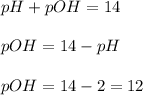
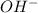 concentration.
concentration.
![pOH=-\log [OH^-]](/tpl/images/0012/3146/1fac1.png)
![12=-\log [OH^-]](/tpl/images/0012/3146/d45ca.png)
![[OH^-]=1.0\times 10^{-12}M](/tpl/images/0012/3146/83579.png)