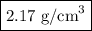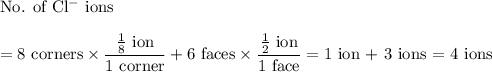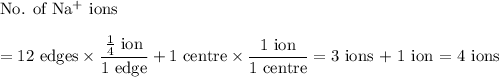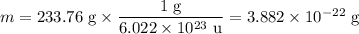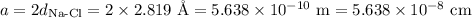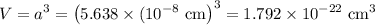Chemistry, 24.06.2019 18:10 makikorising1226
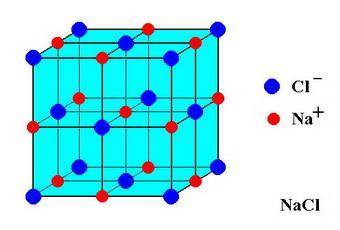
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent chloride ion is 2.819 angstroms. calculate the density in g/cm3of an ideal nacl crystal from this information and what you learned from this lab. hints: to calculate mass, determine how many equivalent ions are in a unit cell. to determinevolume of the unit cell, start by determining the length of on side of the unit cell.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent c...
Questions in other subjects:


Mathematics, 02.04.2020 23:59


Spanish, 02.04.2020 23:59

History, 02.04.2020 23:59


Mathematics, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

Social Studies, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59