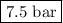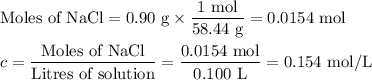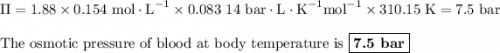Chemistry, 24.06.2019 18:10 carlalopezelox2244
Intravenous, or iv, solutions used in medicine must exert the same osmotic pressure as blood to prevent a net flow of water into or out of the blood cells. the proper concentration for an intravenous nacl solution is 0.90 g nacl per 100. ml of solution (sometimes referred to as 0.90% m/v). if the van't hoff factor of nacl is =1.8, what is the osmotic pressure of blood at body temperature, 37 ∘c?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1


You know the right answer?
Intravenous, or iv, solutions used in medicine must exert the same osmotic pressure as blood to prev...
Questions in other subjects:

Mathematics, 27.04.2021 18:20


Mathematics, 27.04.2021 18:20


Arts, 27.04.2021 18:20



Mathematics, 27.04.2021 18:20


Mathematics, 27.04.2021 18:20