
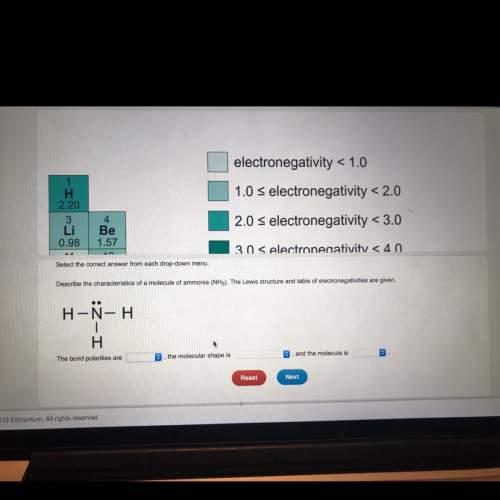
15 ! describe the characteristics of a molecule of ammonia (nh3). the lewis structure and table of electronegative are given. the bond polarities are blank, the molecular shape is blank 2, and the molecule is blank 3. blank 1 options: nonpolar, polar blank 2 options: bent, linear, tetrahedral, trigonometry planar, trigonal pyramidal. blank 3 options: nonpolar, polar each blank has one option.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

You know the right answer?
15 ! describe the characteristics of a molecule of ammonia (nh3). the lewis structure and table of...
Questions in other subjects:

Arts, 15.10.2019 17:30

Chemistry, 15.10.2019 17:30

Physics, 15.10.2019 17:30


Mathematics, 15.10.2019 17:30


Advanced Placement (AP), 15.10.2019 17:30

History, 15.10.2019 17:30

Computers and Technology, 15.10.2019 17:30

English, 15.10.2019 17:30



