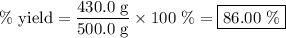Chemistry, 25.06.2019 08:00 saadizak7098
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction meant to fill an order for a customer who would like to purchase 500.0g of ammonia. when the reaction is complete, the company finds they produced only 430.0g of ammonia. what it their percent yield for that reaction? 70.00%86.00%116.3%93.00%

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction...
Questions in other subjects:



Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30


Mathematics, 21.01.2021 21:30

Advanced Placement (AP), 21.01.2021 21:30

Mathematics, 21.01.2021 21:30

Mathematics, 21.01.2021 21:30