Chemistry, 25.06.2019 17:10 sweetbri7p5v6tn
During a laboratory experiment, a 3.81-gram sample of nahco3 was thermally decomposed. in this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. after decomposition, the sample weighed 2.86 grams. calculate the percentage yield of carbonic acid for the reaction. describe the calculation process in detail. nahco3 → na2co3 + h2co3

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
During a laboratory experiment, a 3.81-gram sample of nahco3 was thermally decomposed. in this exper...
Questions in other subjects:

Mathematics, 15.10.2019 09:30





Biology, 15.10.2019 09:30


English, 15.10.2019 09:30

English, 15.10.2019 09:30

Mathematics, 15.10.2019 09:30

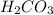 is, 24.44 %
is, 24.44 %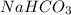 .
.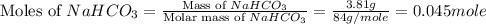
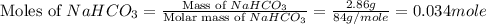
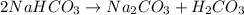
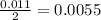 mole of
mole of 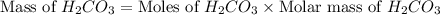
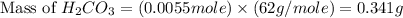
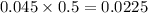 mole of
mole of