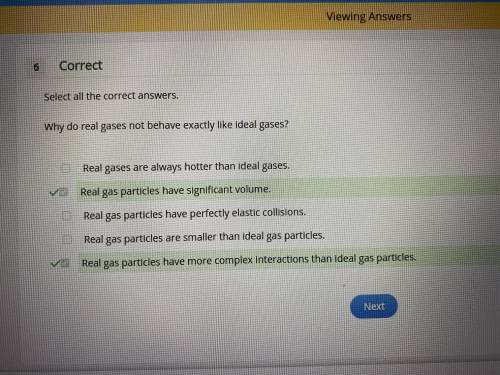
Select all the correct answers. why do real gases not behave exactly like ideal gases? real gases are always hotter than ideal gases. real gas particles have significant volume. real gas particles have perfectly elastic collisions. real gas particles are smaller than ideal gas particles. real gas particles have more complex interactions than ideal gas particles.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
You know the right answer?
Select all the correct answers. why do real gases not behave exactly like ideal gases? real gases a...
Questions in other subjects:






History, 08.04.2020 04:47



Computers and Technology, 08.04.2020 04:47

English, 08.04.2020 04:47