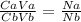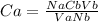Chemistry, 26.06.2019 11:00 anyaimartinez1901
25.0 ml of 0.212 m naoh is neutralized by 13.6 ml of an hcl solution. the molarity of the naoh solution is

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2


Chemistry, 23.06.2019 10:30, villarrealc1987
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
25.0 ml of 0.212 m naoh is neutralized by 13.6 ml of an hcl solution. the molarity of the naoh solut...
Questions in other subjects:




Mathematics, 20.03.2020 09:44