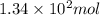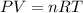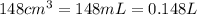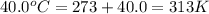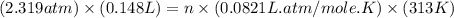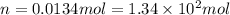Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2


Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
At 40.0°c, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm is 235 kpa. how...
Questions in other subjects:





Biology, 03.11.2019 00:31



English, 03.11.2019 00:31

History, 03.11.2019 00:31