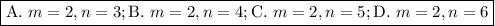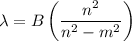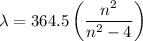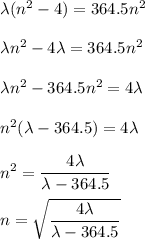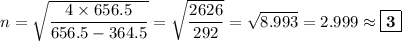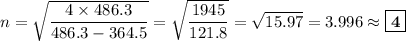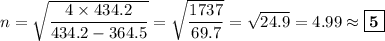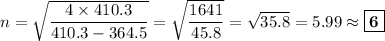Determine the balmer formula n and m values for the wavelength 656.5 nm. possible choices: m= 1 n= 2 m= 2 n= 3 m= 3 n= 4 m= 2 n= 5 part b determine the balmer formula n and m values for the wavelength 486.3 nm. possible choices: m= 1 n=2 m= 2 n=3 m= 1 n=4 m= 2 n=4 part c determine the balmer formula n and m values for the wavelength 434.2 nm. possible choices: m= 1 n= 4 m= 2 n= 4 m= 3 n= 4 m= 2 n= 5 part d determine the balmer formula n and m values for the wavelength 410.3 nm. possible choices: m= 2 n= 4 m= 2 n= 5 m= 3 n= 4 m= 2 n= 6

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
Determine the balmer formula n and m values for the wavelength 656.5 nm. possible choices: m= 1 n=...
Questions in other subjects:



Chemistry, 11.02.2020 05:05





Social Studies, 11.02.2020 05:05