
Chemistry, 26.06.2019 18:50 christianfielding336
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing
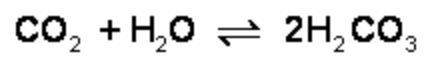

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions in other subjects:




Mathematics, 27.06.2019 21:30





Physics, 27.06.2019 21:30

Mathematics, 27.06.2019 21:30



