
Chemistry, 27.06.2019 22:50 Cheflulu5727
How many milliliters of a 1.25 molar hydrochloric acid (hcl) solution would be needed to react completely with 60.0 grams of calcium metal? (2 points) ca (s) + 2hcl (aq)yields cacl2 (aq) + h2 (g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1


Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
How many milliliters of a 1.25 molar hydrochloric acid (hcl) solution would be needed to react compl...
Questions in other subjects:










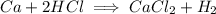
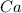 and
and  for the complete reaction is 1:2.
for the complete reaction is 1:2.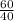 = 1.5 mol
= 1.5 mol = 3.0 mol
= 3.0 mol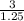 = 2400 mL
= 2400 mL

