
Chemistry, 28.06.2019 03:00 aidentrooper8629
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric acid. if the percent yield of the reaction was 82.15%, what was the actual amount of calcium chloride formed? caco3 + hcl → cacl2 + co2 + h2o 105.3 grams 101.1 grams 95.6 grams 86.5 grams

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric ac...
Questions in other subjects:



Mathematics, 22.11.2019 17:31

Chemistry, 22.11.2019 17:31






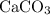 ?
?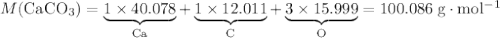 .
.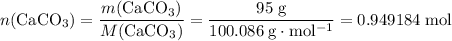 .
.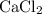 will be produced?
will be produced?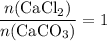 .
.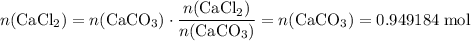 .
. of
of 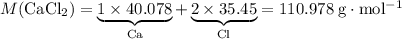 .
.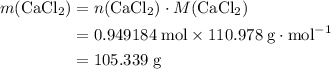 .
.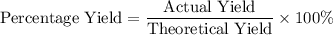 .
. .
.

