Chemistry, 29.06.2019 10:20 gonzalesrosalinda66
During a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal at 290.0 k and 1.3 atm. what was the volume of o2 used during the experiment? 3o2 + 4al → 2al2o3 a. 10.10 liters b. 11.90 liters c. 12.51 liters d. 15.55 liters

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, rileyallen4186pd5tgy
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
During a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal...
Questions in other subjects:

Biology, 27.08.2019 14:50

Social Studies, 27.08.2019 14:50

Physics, 27.08.2019 14:50



History, 27.08.2019 14:50

English, 27.08.2019 14:50


Chemistry, 27.08.2019 14:50


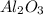 = 44.18 g
= 44.18 g