
Chemistry, 29.06.2019 11:20 gabriel5575
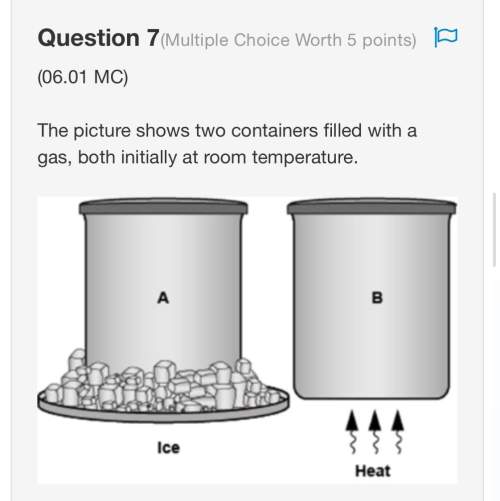
** worth 30 points plus brainliest ** the picture shows two containers filled with a gas, both initially at room temperature. two equally sized containers are shown with tight lids. the container on the left is labeled a and the one on the right is labeled b. below container b, its written heat and below container a its written ice. ice cubes are shown below container a. which statement is correct? a. the gas particles in both containers have the same average kinetic energy because they have equal number of particles. b. the gas particles in both containers have the same average kinetic energy because they have the same volume. c. the average kinetic energy of the gas particles is greater in container a because it has a lower temperature. d. the average kinetic energy of the gas particles is greater in container b because its particles move faster.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

You know the right answer?
** worth 30 points plus brainliest ** the picture shows two containers filled with a gas, both ini...
Questions in other subjects:


English, 09.12.2020 01:20

Mathematics, 09.12.2020 01:20







English, 09.12.2020 01:20




