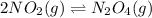Chemistry, 29.06.2019 21:50 dooboose15
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat and the formation of nitrogen dioxide absorbs heat. if the reaction is at equilibrium and the temperature increases, what will the effect be? a. nitrogen dioxide absorbs heat and changes from gas to liquid. b. the equilibrium will shift so that there is more nitrogen dioxide. c. the equilibrium will shift so that there is more dinitrogen tetroxide. d. dinitrogen tetroxide absorbs heat and changes from gas to liquid.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2


Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat...
Questions in other subjects:

Biology, 30.07.2019 10:30


Chemistry, 30.07.2019 10:30


Business, 30.07.2019 10:30

Social Studies, 30.07.2019 10:30



Chemistry, 30.07.2019 10:30