

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Determine the enthalpy change of the following reaction: co + h2o -> h2 + co2 given enthalpies...
Questions in other subjects:


Chemistry, 12.02.2021 20:50

Mathematics, 12.02.2021 20:50




Mathematics, 12.02.2021 20:50


Biology, 12.02.2021 20:50

Mathematics, 12.02.2021 20:50

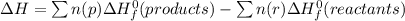
![\Delta H = [1\Delta H_{f}^{0}(H2)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CO)+1\Delta H_{f}^{0}(H2O)]](/tpl/images/0034/1721/21403.png)


