Chemistry, 04.01.2020 14:31 battlemarshmell
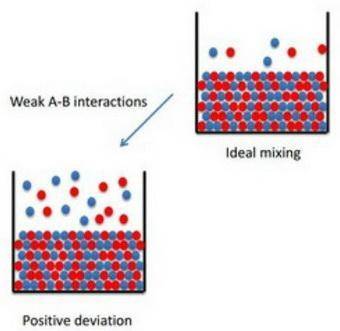
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. the resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. if, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. an actual vapor pressure greater than that predicted by raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by raoult's law is a negative deviation. part a imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. will this solution deviate positively from, deviate negatively from, or ideally follow raoult's law? '

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 to...
Questions in other subjects:


History, 26.07.2019 05:30

Biology, 26.07.2019 05:30



History, 26.07.2019 05:30



History, 26.07.2019 05:30